eQuilibrator: Balancing Reactions
The more I study biology the more I realize that I am not a chemist. I studied computer science and applied math in college and made my way into biology slowly. After 8 years, I am comfortable with biology. But I am not a chemist. At least not yet. But when you pursue biology, a whole ton of chemistry comes up. Chemistry training seems to enable people to visualize molecules quickly and imagine reactions between them. For example, it may be obvious to someone trained as a chemist or biochemist that you need to dehydrate glucose to make gluconate.
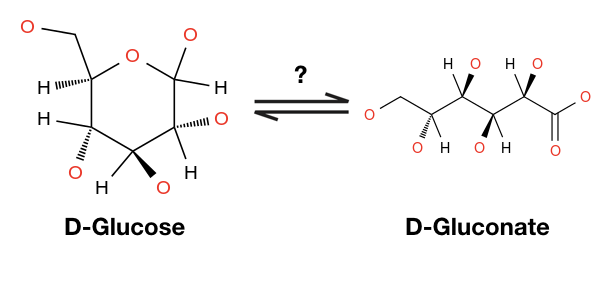
But I am not a chemist and so I use eQuilibrator. If you search “glucose = gluconate” in eQuilibrator the site will notice that these molecules differ in their atomic composition by a water molecule. Since water is such a common thing to forget, we made this one easy on you. You can click “Balance with H2O” and eQuilibrator will automatically correct this imbalance.
But now eQuilibrator notices that glucose has two more electrons than gluconate + H2O, i.e that “glucose = gluconate + H2O” is a half-reaction. Electrons are not soluble so the electrons in this half-reaction have to go somewhere. Usually they will go to one of the common biological electron carriers like NAD(P)H, quinones, flavins, ferredoxins, etc. In particular, you can click “Balance with NAD+/NADH” and eQuilibrator will add NAD+ and NADH to the reaction in the correct stoichiometry so that electrons and atoms are all balanced. Now that you’ve got a balanced reaction we can give you that ΔrG’ estimate you came for.